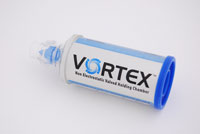
Objective:
Design an efficient, low-cost, FDA approved, inhalation therapy drug delivery device for volume production that:
- Outperforms competitive devices.
- Reduces drug plate-out due to static charge.
- Improves drug dispersion in the air steam.
- Is usable by a broad range of patients.
Engineering Tools and Prototype Fabrication
- Acquire and evaluate rigid and elastomeric parts using stereolithography.
- Develop Materials Selection Criteria.
- Anodized aluminum alloy to meet FDA toxicity requirements.
- Chemical resistant, steam resistant, transparent thermoplastic, thermoset elastometer, adhesive for metal/plastic bonded joint and adhesive-backed label materials.
Design Features:
- Employs an ATI designed, one piece two-way valve.
- Product design uses a snap-together assembly for easy cleaning.
- Mates with metered dose inhalers and accessories.
- Impact resistant.
- Can be sterilized in a steam autoclave.
Manufacturing Support:
- Identify metal part supplier.
- Identify molding supplier for thermoplastic and thermoset parts.
- Exported digital 3-D files for tool path and tool build.
- Evaluated steam resistance for thermoplastic materials, adhesives, and labels.
- Design and support fabrication of test fixtures.
![]()